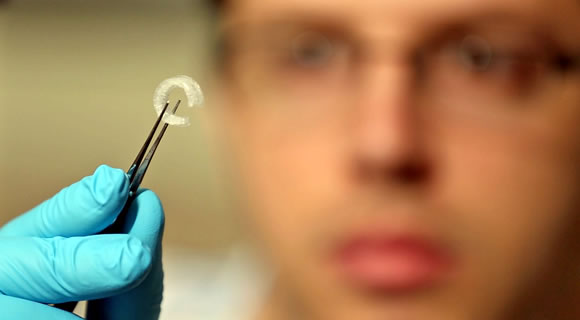
Services
CLINADE provides the clinical research infrastructure support for principal investigators to make medical research less demanding. We employ physicians as independent contractors to conduct and oversee the clinical trial, while serving as a principal investigator representative to the sponsoring companies. We ensure that every clinical trial we conduct is in accordance with ICH Good Clinical Practices and the FDA Code of Federal Regulations.
Our goal is to make medical research a seamless process for investigators by assisting and navigating physicians through the seemingly
daunting process of clinical trials.